Girbl Group
The passage of circulating immune cells through blood vessel walls is a key step in the establishment of effective immune responses and a critical component in the development of multiple inflammatory diseases. Therefore, a detailed understanding of the interactions between immune cells and the blood vasculature is essential for our basic understanding of the immune system and for the development of novel therapies for pathological conditions, including autoimmune diseases, myocardial infarction and stroke.
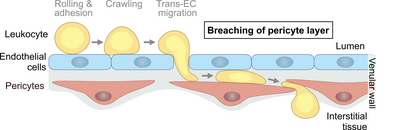
Venular pericytes in immunity
In order to enter extravascular tissue compartments, leukocytes pass the venular endothelium in a defined cascade of adhesive events. However, much less is known about how leukocytes overcome the second cellular component of venular walls: the pericyte sheath. Our previous work has shown that pericyte-derived chemokines provide essential guiding cues for neutrophil migration into inflamed tissues (Girbl et al., Immunity 2018).
Our current research aims to elucidate how leukocytes interact with venular pericytes during innate and adaptive immune responses in vivo. In this context, we are interested in the molecular mechanisms mediating lymphocyte migration through the pericyte network and the potential immunoregulatory role for pericytes during physiological inflammation as well as pathological conditions. To address our research questions, we utilize high resolution intravital confocal and multiphoton microscopy, transgenic mouse models, RNA sequencing and reductionist in vitro cell migration assays.
Joulia R, Guerrero-Fonseca IM, Girbl T, Coates JA, Stein M, Vázquez-Martínez L, Lynam E, Whiteford J, Schnoor M, Voehringer D, Roers A, Nourshargh S, Voisin MB. Neutrophil breaching of the blood vessel pericyte layer during diapedesis requires mast cell-derived IL-17A. Nat Commun. 2022 Nov 17;13(1):7029. doi: 10.1038/s41467-022-34695-7.
Barkaway A, Rolas L, Joulia R, Bodkin J, Lenn T, Owen-Woods C, Reglero-Real N, Stein M, Vázquez-Martínez L, Girbl T, Poston RN, Golding M, Saleeb RS, Thiriot A, von Andrian UH, Duchene J, Voisin MB, Bishop CL, Voehringer D, Roers A, Rot A, Lämmermann T, Nourshargh S. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity. 2021 Jul 13;54(7):1494-1510.e7. doi: 10.1016/j.immuni.2021.04.025.
Owen-Woods C, Joulia R, Barkaway A, Rolas L, Ma B, Nottebaum AF, Arkill KP, Stein M, Girbl T, Golding M, Bates DO, Vestweber D, Voisin MB, Nourshargh S. Local microvascular leakage promotes trafficking of activated neutrophils to remote organs. J Clin Invest 2020; p133661, doi: 10.1172/JCI133661.
Girbl T, Lenn T, Perez L, Rolas L, Barkaway A, Thiriot A, Del Fresno C, Lynam E, Hub E, Thelen M, Graham G, Alon R, Sancho D, von Andrian UH, Voisin MB, Rot A, Nourshargh S. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018;49(6):p1062-1076, doi: 10.1016/j.immuni.2018.09.018.
Gutjahr JC, Szenes E, Tschech L, Asslaber D, Schlederer M, Roos S, Yu X, Girbl T, Sternberg C, Egle A, Aberger F, Alon R, Kenner L, Greil R, Orian-Rousseau V, Hartmann TN. Microenvironment-induced CD44v6 promotes early disease progression in chronic lymphocytic leukemia. Blood 2018;22;131(12):p1337-1349, doi: 10.1182/blood-2017-08-802462.
Schulz-Fincke AC, Tikhomirov AS, Braune A, Girbl T, Gilberg E, Bajorath J, Blaut M, Nourshargh S, Gütschow M. Design of an Activity-Based Probe for Human Neutrophil Elastase: Implementation of the Lossen Rearrangement To Induce Förster Resonance Energy Transfers. Biochemistry 2018;57(5):p742-752, doi: 10.1021/acs.biochem.7b00906.
Ganghammer S, Hutterer E, Hinterseer E, Brachtl G, Asslaber D, Krenn PW, Girbl T, Berghammer P, Geisberger R, Egle A, Zucchetto A, Kruschinski A, Gattei V, Chigaev A, Greil R, Hartmann TN. CXCL12-induced VLA-4 activation is impaired in trisomy 12 chronic lymphocytic leukemia cells: a role for CCL21. Oncotarget 2015;20;6(14):p12048-60, doi: 10.18632/oncotarget.3660.
Girbl T, Lunzer V, Namberger K, Greil R, Hartmann TN. The CXCR4 and adhesion molecule expression of CD34+ hematopoietic cells mobilized by on-demand addition of plerixafor to G-CSF. Transfusion 2014;54(9):p 2325-35, doi: 10.1111/trf.12632.
Asslaber D, Grossinger EM, Girbl T, Hofbauer SW, Egle A, Weiss L, Greil R, Hartmann TN. Mimicking the microenvironment in chronic lymphocytic leukaemia - where does the journey go? Br J Haematol 2013;160:p 711-714, doi: 10.1111/bjh.12151.
Girbl T, Hinterseer E, Grossinger EM, Asslaber D, Oberascher K, Weiss L, Hauser-Kronberger C, Neureiter D, Kerschbaum H, Naor D, Alon R, Greil R, Hartmann TN. CD40-mediated activation of chronic lymphocytic leukemia cells promotes their CD44-dependent adhesion to hyaluronan and restricts CCL21-induced motility. Cancer Research 2013;73:p 561-570, doi: 10.1158/0008-5472
Brachtl G, Sahakyan K, Denk U, Girbl T, Alinger B, Hofbauer SW, Neureiter D, Hofbauer JP, Egle A, Greil R, Hartmann TN. Differential bone marrow homing capacity of VLA-4 and CD38 high expressing chronic lymphocytic leukemia cells. PLoS One 2011;6:p e23758, doi: 10.1371/journal.pone.0023758.
Gadermaier G, Hauser M, Egger M, Ferrara R, Briza P, Santos KS, Zennaro D, Girbl T, Zuidmeer-Jongejan L, Mari A, Ferreira F. Sensitization prevalence, antibody cross-reactivity and immunogenic peptide profile of Api g 2, the non-specific lipid transfer protein 1 of celery. PLoS One 2011;6:p e24150, doi: 10.1371/journal.pone.0024150.
Gadermaier G, Egger M, Girbl T, Erler A, Harrer A, Vejvar E, Liso M, Richter K, Zuidmeer L, Mari A, Ferreira F. Molecular characterization of Api g 2, a novel allergenic member of the lipidtransfer protein 1 family from celery stalks. Mol Nutr Food Res 2011;55:p 568-577, doi: 10.1002/mnfr.201000443.
Gadermaier G, Harrer A, Girbl T, Palazzo P, Himly M, Vogel L, Briza P, Mari A, Ferreira F. Isoform identification and characterization of Art v 3, the lipid-transfer protein of mugwort pollen. Mol Immunol 2009;46:p 1919-1924, doi: 10.1016/j.molimm.2009.03.021.
Dr. Tamara Girbl
Josef-Schneider-Str. 2

Lara Schober
Josef-Schneider-Str. 2

Tugce Cimen
Josef-Schneider-Str. 2

Dr. Marilia Fernandes Manchope
Josef-Schneider-Str. 2

Current position
Group leader at the Rudolf Virchow Center of the University of Wuerzburg (since June 2020)
Research Experience
2018 – 2020 Postdoctoral Researcher with Prof. Michael Sixt, Institute of Science and Technology (IST) Austria
2014 – 2018 Postdoctoral Fellow of the British Heart Foundation with Prof. Sussan Nourshargh, Queen Mary University of London, United Kingdom
2009 – 2012 PhD with Prof. Tanja Hartmann and Prof. Richard Greil, University Clinics Salzburg, Austria
Education
2013 PhD (Dr. rer. nat.) in Genetics and Molecular Biology, University of Salzburg, Austria
Major Fellowships and Awards
2021 Start-up grant from the University of Würzburg
2019 Servier Award for Microcirculation
2017 Young Investigator Award of the European Society for Microcirculation
2016 Travel & presentation awards from the Gordon Research Conference on Chemotactic Cytokines
2016 Travel fellowship, European Chemokine and Cell Migration Meeting
2014 – 2018 BHF Immediate Postdoctoral Fellowship
2014 – 2016 Marie Curie Co-Fund Postdoctoral Fellowship
2013 Sanofi publication award
2013 Wilhelm Türk publication award from the Austrian Society for Hematology & Oncology
2009 – 2013 International PhD Programme Immunity in Cancer and Allergy (FWF, Austria)