Research Focus
In spite of the availability of preventive strategies, infectious diseases continue to be a major threat worldwide. Therefore, there is a demand for continuous development of anti-infective or immuno-therapeutic strategies, in particular for conditions where conventional interventive means are not available, prohibited or fail.
To control infectious diseases, novel interventive strategies should therefore effectively modulate
1) innate and adaptive immune responses and/or
2) tissue and cell compartment specific autonomous metabolic parameters all - of which operate to limit (or in some instances also promote) both pathogen spread and tissue damage.
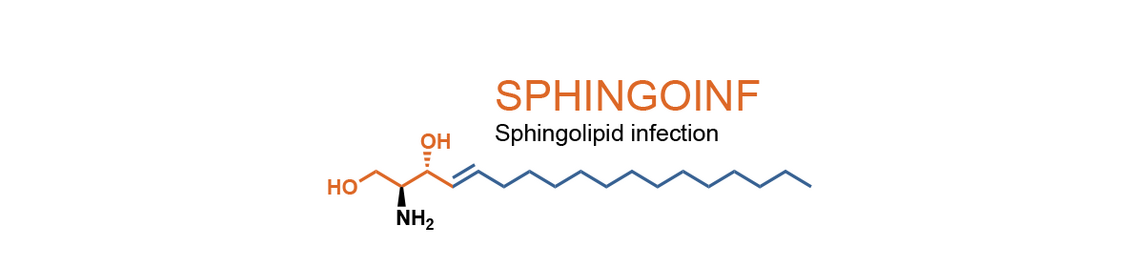
Common denominators of these cellular processes are dynamic alterations in membrane metabolism. This efficiently defines compartmentalization of the repertoire of host and immune cell receptors, associated signaling pathways, cytoskeletal dynamics and effector mechanisms. Because sphingolipids are major components of membranes, sphingolipid biosynthesis and metabolism and availability of their signaling inert or bioactive species substantially affects the biophysical properties of membranes and the subcellular redistribution of receptors and signaling complexes. This may essentially regulate pathogen uptake and handling at a cellular and organismic level as well as survival and activity of immune cells where they shape the magnitude and quality of the individual cellular compartments acting to control a given pathogen.
Targeted intervention of sphingolipid turnover has proven to be a successful strategy in inflammation, but its potential as a target in controlling infectious diseases at the level of metabolism and immune controls requires further definition. Therefore, the RTG initiative aims to identify and validate targets for novel anti-infective or immunotherapeutic strategies targeting infectious diseases at the level of modulation of the sphingolipid metabolism.